RELIQUEFACTION PLANT
1 General theory
1.1 Description
When the cargo temperature is below ambient temperature, a certain amount of heat will leak into the cargo through the tank insulation (i.e. heat ingress). The temperature of a liquid gas cargo is always equal to the boiling temperature at the actual pressure, and any heat ingress will cause evaporation of the cargo. By constant tank pressure, the heat ingress is accumulated in the vapour formed by boiling, and the main purpose for the reliquefaction plant is to remove and reliquefy this vapour, and to return the condensate to the cargo tanks, otherwise the heat ingress will result in increased temperature and pressure.
In a refrigeration cycle the refrigerant circulates from the evaporator through the compressor to the condenser and back to the evaporator .
The heat removed in the evaporator by boiling off refrigerant is taken from the surroundings. In the compressor an additional amount of heat is conveyed to the refrigerant by the compression process. The total heat is then removed from the refrigerant in the condenser .
The principle of operation of the 131b16b reliquefaction plant onboard this ship is as described above. The cargo tanks act as evaporators and the cargo as refrigerant, and the seawater are the coolant for the condenser .
Increasing difference between evaporating and condensing pressure reduces the capacity of the cargo compressor .
A large compression ratio may also result in too high a discharge temperature. These problems may be avoided by dividing the compression into two or three stages with intercooling.
1.2 Vapour pressure
When water is heated in an open vessel, air bubbles will be formed and bubble up through the water. These bubbles are dissolved air, separated out due to the temperature of the water increasing. The rise in temperature continues until the boiling point is reached. Before boiling starts, water evaporates from the water surface, and the evaporation increases as the temperature rises. At the boiling point, evaporation takes place throughout the water. The vapour bubbles up through the water and the temperature rise ceases, as the entire heat supply is used for the evaporation.
The vapour will displace the air above the water, and the vapour temperature will be the same as the water temperature, i.e. the vapour is saturated as no more water can be absorbed in the vapour .
The boiling point rises when the pressure increases. The boiling point is therefore, not the same at sea level as on a summit. To boil food in a pressure boiler is quickly done, as the pressure, and thereby the temperature, is high. Water may boil at room temperature if the pressure is decreased sufficiently. Boiling is therefore, not a function of temperature only. Depending on the pressure and the medium, boiling may occur at very low temperatures.
If liquid is filled into a closed vessel, and the space above the liquid surface is filled with vapour boiled off from the liquid, there will always be one particular pressure that corresponds to one particular temperature when there is equilibrium between the liquid and the vapour phases. The method shown in section 7, Fig. 7.7 is often used to express the pressure - temperature relationship. The pressures are plotted logarithmically versus the temperatures. The vapour pressure curves for the various substances are approximately rectilinear. This method gives more exact values at low pressure than the use of linear scales.
1.3 Mollier diagram (log p -h diagram)
As mentioned under Vapour Pressure, the water temperature increases when heat is supplied until the boiling point is reached. One particular amount of heat supplied, expressed in Joule (or kcal) per kilo of water, should therefore correspond to one particular temperature. However, when the temperature comes to the boiling point, the temperature can no longer be used as a measure of heat amount supplied per kilo of water, as the supplied heat is used to evaporate the water .
A particular amount of heat is necessary to evaporate one kilo of water. This heat amount, which is called the latent heat of evaporation, decreases when the boiling point rises. When the vapour is cooled down, condensation takes place, and the latent heat of evaporation is liberated. To facilitate the calculations in which these heat amounts appear, the term enthalpy with the notation 'h' (or 'i') and designation J/kg (or kcal/kg) is used. Enthalpy is also frequently called heat content, and expresses the amount of heat present in each kilo of the substance in question under the given conditions.
The enthalpies can be tabulated, and usually tables are used to find the enthalpies for saturation conditions. Tables for other than saturation conditions would, however, be very large and impractical, and for most substances, therefore, diagrams are made up in which enthalpies are plotted along the other. Such a diagram is called a Mollier-diagram, p -i diagram or log p -h diagram, as the pressure is given logarithmically.
The figure below shows part of a log p -h diagram for Propane. The arched line running from the bottom left-hand corner upwards towards the right is called the saturated liquid line. This line turns almost vertically downwards in the middle of the diagram. This part of the line is called the saturated vapour line (commonly referred to as the saturation line).
The area between this line and the saturated liquid line indicated mixtures of gas and liquid. The area to the left of the saturated liquid line indicated sub-cooled liquid, and the area to the right of the saturated vapour line indicates superheated vapour .
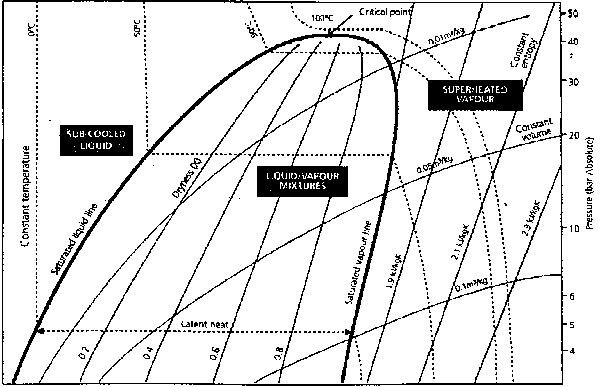 |
The distance between the saturated liquid line and the saturated vapour line corresponds to the heat of evaporation. As can be seen in the diagram, the heat of evaporation decreases when the pressure increases, until it is zero at the critical point.
The lines bending to the right are lines for constant volume.
The course of the lines running through one temperature is illustrated by the dotted line that represents 0 oC. The stippled lines run through points with constant entropy and indicate the increase in heat content when the gas is compressed. The curves show the adiabatic compression. The actual compression course differs from this curve due to varied losses.
The doubled stippled line in the area between the saturated liquid line and the saturated vapour line run through points having the same quality (x). The x-curves indicate the ratio between gas and liquid, i.e., X = 0.6 means that 60 % by weight of the substance is gaseous and 40 % is liquid.
1.4 Pressure increase due to presence of ethane and inert gas
Pure propane is unlikely to be transported in bulk. The cargo usually consists of a mixture of hydrocarbons, mainly propane (70-98 vol %), butane (5-20 vol %), and ethane (1-3 vol %). Such a mixture is usually called Commercial Propane.
Ethane will increase the cargo vapour pressure, and butane will decrease the vapour pressure relative to pure propane. At a relatively low concentration of butane, the effect from this medium is insignificant compared to that of ethane.
Due to the volatility of ethane, the gas phase above commercial propane contains more ethane than the liquid. The vapour sucked into the compressors will therefore have a higher ethane concentration than the liquid cargo. The composition of the condensate returned to the tanks must obviously be equal to the concentration of the vapour sucked off.
The mechanism of this vapour enrichment is shown in Fig. 7.5 section 7, which is called an equilibrium diagram for mixtures of propane and ethane at a certain pressure. As can be seen in the diagram, a mixture containing 4 mole % ethane in the liquid phase will contain about 24 mole % ethane in the vapour phase at 1.0 bara (almost equal to 1.0 ata). When dimensioning the reliquefaction plant, it must be taken into consideration that the gas with the high ethane content is to be condensed. If this gas, containing 24 mole % of ethane, was to be condensed at 1.0 bara, the condensing temperature would, according to Fig 7.5 section 7, be as low as minus 65 oC. In Fig 7.8 section 7 are shown pressure temperature curves for propane/ ethane mixtures with different ethane contents in the liquid phase.
Assuming that the condensing temperature is 40 oC, the diagram gives a condensing pressure of 22 bara, provided the ethane content in the liquid phase is 24 mole %.
The saturation pressure for pure propane at 40 oC is 14 bara. The conclusion is that the presence of extremely volatile hydrocarbons increases the condensing pressure.
The ratio between the concentrations of the gas and the liquid phases is not constant, but
varies in accordance with the saturation temperature, as shown in Fig. 7.9 section 7. The saturation temperatures are plotted along the horizontal axis, and the concentration in the gas phases is plotted along the vertical axis. The curves run through points having the same concentration in the liquid phase. For instance, the 2.5 mole percent in the liquid at equilibrium with the gas phase, gives 7.5 mole % ethane in the gas phase at plus 20 oC, and 12.5 mole % at minus 30 oC.
Also the presence of inert gas or air in the system causes an increased pressure in the condenser. These gases will be led from the tanks ( for instance the first cargo after gas freeing) and will collect in the condensers. It is not possible to condense neither inert gas nor air at the pressure and temperature conditions prevailing in the condensers of a gas carrier .
The partial pressures for these gases must therefore be added to the vapour pressure of for instance propane. If the condensing pressure becomes higher than the saturation pressure, a bleed off of non-condensable gases to the tanks can be carried out. When the non- condensable gases are blown into the liquid cargo, the greatest part of the gases will be solved in the liquid. Greater amounts of non-condensable gases in the condensers have to be blown off to the vent mast or to shore.
2 Plant description( See also makers instruction manual )
2.1 General
The ship is equipped with a direct three-stage reliquefaction plant where the cargo is used as refrigerant. The reliquefaction unit is shown on H-KSEs piping diagram Fig.
The plant consists of four identical units, each consisting of one 3-stage cargo compressor, one intermediate cooler, one cargo condenser against seawater (with integrated receiver), control valves and connecting piping with valves and strainers etc.
2.2 Operation Modes
The main operation modes for the reliquefaction plant are One-stage compression.
Two-stage compression, with intercooling. Three-stage compression, with intercooling.
The choice of operation mode is decided by Thermodynamic properties of the cargo. Requirement for cooling capacity . Seawater temperature.
Our recommendations for operation modes for the different cargoes are given in section 9 COOLING. Please also refer to maker's instruction manual.
A brief process description for one-, two- and three-stage operation is given below
2.3 Process Description
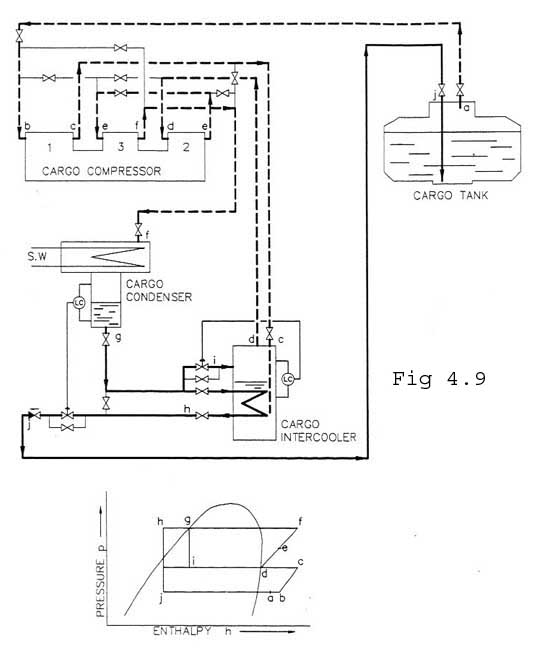
Due to heat transmission, a heat quantity = Qtrt is led into the tank. The compressors suck vapour with a certain heat quantity from the tank (state 'a' in Fig. 4.7 to 4.9).
Fig 4.7 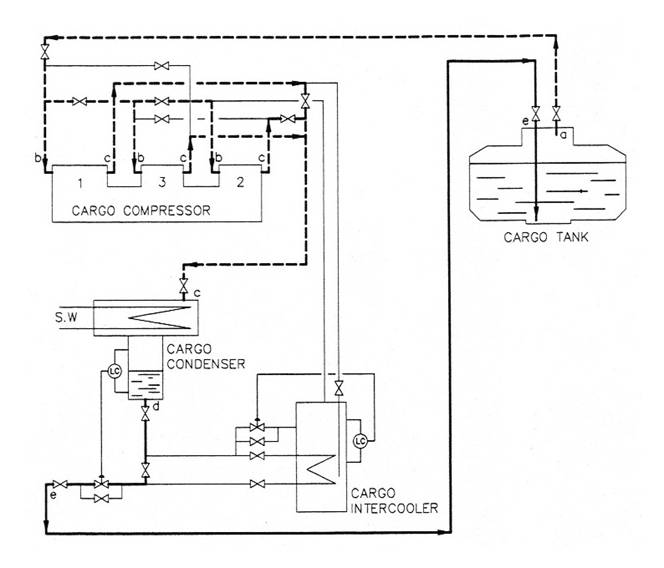
On its way from the tank to the compressor, the temperature in the gas increases due to heat ingress through the pipe watts, Qtrp.
At l-stage compression, the three cylinders are working in parallel. During compression (b-c in Fig. 4.7) a heat amount = Qcomp is supplied to the gas, resulting from the power supplied to the compressor motor. The temperature of the gas will increase, and the gas will be considerably superheated.
In the condenser the gas is first cooled down from 'c', until the saturation temperature is reached at the actual pressure, which depends on the seawater temperature. Further, the gas is condensed at constant pressure and temperature, until the saturated liquid line ( d) is reached. The heat amount transferred from the gas to the seawater during the changing from state 'c' to state 'd' is Qc'. ,
The regulating valve reduces the pressure from the condensing- to the tank pressure, 'd' - 'e'. As there is no supply or giving of heat during this part of the process, the enthalpy does not change.
The regulating valve controls the level in the receiver. The regulator provides a liquid trap to prevent a flow of non-condensed gases back to the tank, which would reduce the efficiency of the plant.
l-stage compression is normally used for condensation of the 'warmer' LPG cargo butane (Fig. 4.7).
At 2-stage compression, the 2nd and 3rd stages are run in parallel, but in series with the 1st stage. The plant is operating with intercooling after -1st stage, and with subcooling of condensate. Used for propane at low seawater temperatures. The operation mode is shown in Fig. 4.8.
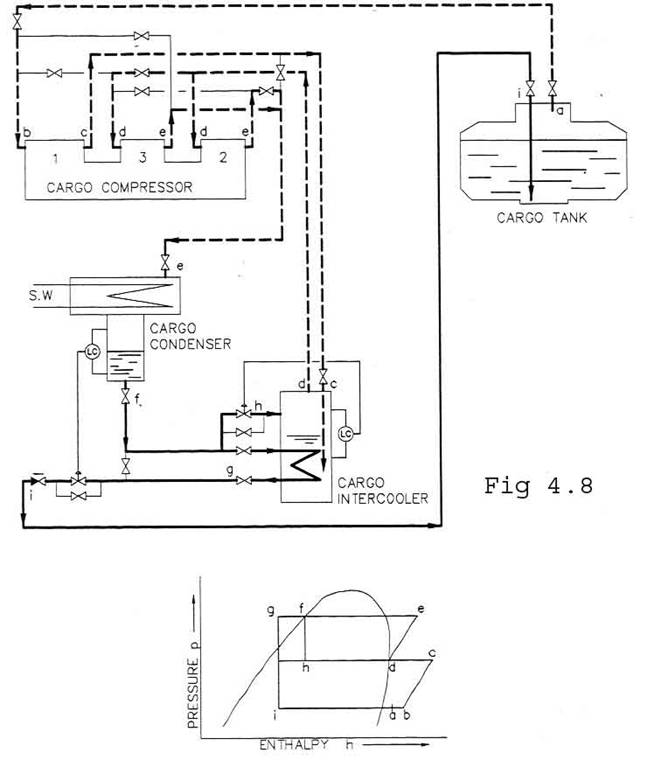
The gas is compressed in the LP-cylinder (b-c), cooled in the cargo intercooler (c-d) and compressed in the HP-cylinders (d-e). The condensate is subcooled on the intercooler (f-g) before it is expanded back to the cargo tank. The subcooling is effected by liquid condensate in the intercooler. The condensate level in the intercooler is kept at constant level by feeding from the liquid return line, (f-h).
When operating the intercooler, the main condensate flow is led through a coil inside the intercooler where the condensate is subcooled to approx. 5-10 oC above the saturation temperature in the intercooler .
The condensate is then expanded back to the tanks through the modulating control valve (g- i). The level in the receiver controls this valve.
Part of the liquid in the intercooler will evaporate since it received heat from the LP-cylinder gas and the subcooling described above. Therefore a certain amount of the condensate in the receiver is fed to the intercooler by the modulating control valve.
The intercooler level controls this valve and is only used when the units are operating with intercooling.
When the units are operated without intercooling, the condensate is returned from the receiver directly to the tanks. This operation mode will increase the discharged temperature and reduce the cooling capacity. Operation with intercooling should be preferred.
At 3-stage compression (fig. 4.9), the 2nd and the 3rd stage are running in series. Intercooling and subcooling of condensate as described above. To be used for propane at high seawater temperatures.
The regulating valves are of globe type with pneumatic membrane actuators that are controlled by 3-15 psi air signal supplied by the pressure operated level controllers.
The heat balance for the cargo cycle may be expressed as follows, provided constant temperature of the cargo in the tanks:
Heat leakage to tanks Qtrt
+ Heat leakage to pipes Qtrp
+ Heat transferred during compression Qcomp
= Heat removed in the condenser Qc
3 Cargo compressor (See also makers instruction manual)
3.1 General description
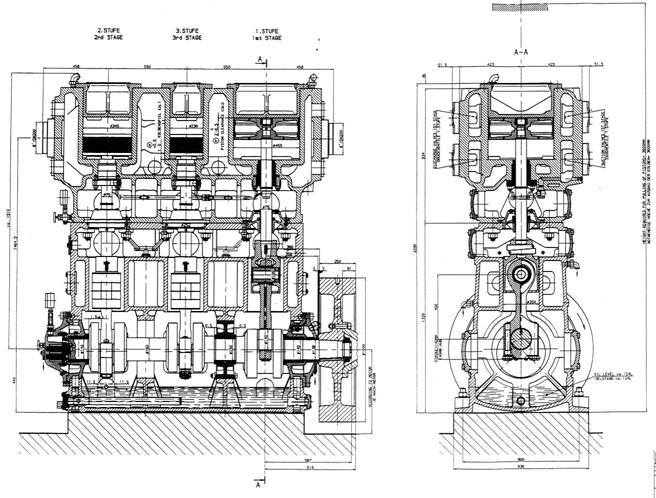
The three cargo compressors are oil-free, three-stage, double-acting piston compressors made by Sulzer, type 3K 140-3A. Each compressor is directly driven by an electric motor at rotating speed 710 rpm.
As the electric motors are located in a separate el-motor room, an oil-filled gas tight stuffing box is arranged for the drive shaft. The level in the oil reservoirs must be checked at intervals, and oil refilled if necessary .The stuffing box is fitted with a temperature indicator . The oil system for lubricating and cooling of bearings and stuffing boxes in the cargo compressors is described in the Sulzer instruction manual- A piping diagram showing the lub. oil arrangement inside the compressor room is shown in section 5.
The glycol system for heating and cooling of the compressor is also described in section 5
The cargo compressors are equipped with capacity regulation. By lifting of suction valves, the capacity may be reduced to 50 %. Handle for capacity regulation is arranged in front of the compressors.
The compressors are started locally, and stopped locally and remotely. For running-in instructions for the cargo compressors, it is referred to Sulzer's manual. The normal start- and stop procedure for the compressors is described in Sulzer’s manual, and also listed below, with some additional instruction.
3.2 Normal Starting
Before leaving the compressor room:
Check that all pneumatic valves are working properly and check that the cargo receiver is not filling up due to ice in the control valves.
Check the lub. oil pressure. normal 3.2- 4.0 bar.
Check the suction-, (intermediate- ) and discharge pressure. Due to the pressure losses in the suction piping. the suction pressure will be slightly below the tank pressure. The discharge pressure is dependent on the seawater temperature. This pressure will correspond to a condensing temperature normally 5-10 oC above the seawater temperature. Higher pressures indicate ineffective cooling (maximum, respectively minimum admissible pressures, see Sulzer's manual).
Check the gas temperatures at suction- and discharge side of the compressor. (Maximum admissible temperatures, see Sulzer's manual). The temperature on the Suction side must be higher than the corresponding dew point of the gas, to avoid condensation ( danger of liquid knock).
Check oil leakage of shaft seal at compressor (maximum 3 drops per minute),
Check glycolled water temperature at compressor outlet. This should be within 35 to 45 oC. Sudden temperature variations must be avoided.
3.3 Continuous Operation
The following should be supervised periodically when compressors are running:
Sulzer recommend to record readings of pressures, temperatures, ampere and voltage in a service log. See example of service log in Sulzer manual.
For further information regarding the compressor please see makers instruction manual as well as Sulzer' s instruction book for the compressors.
In most ports, the seawater is highly corrosive. Therefore the condensers should be flushed as soon as the vessel has left port. Once each year the tubes in the condensers should be inspected. Possible deposits on the tubes will decrease the cooling capacity and should be removed using appropriate tools. For further information regarding the condenser please see makers instruction manual.
A outline drawing and sectional view of the cargo compressor are shown in Fig. 4.12.
The spare part store must always be complete. As soon as one part is in service it must be replaced.
|