Importance and applications of polyelectrolytes/ surfactans complexes
The various names used in detergent industry, in cosmetics and in agroalimentary industry are in fact referring to the molecules of polyelectrolytes and surfactants. For example, the formulae of shampoo or shower gel contain surfactants negatively charged, like Sodium Lauryl Sulfate and polyelectrolytes positively charged, like hydroxyquaternium guars, and many other compounds (Thickening agent, detergent, perfume, anti-vexatious, etc. The same, the chocholate creams are composed among others of proteins from eggs and natural surfactants. Finally, in our body, the membranes of our cells are composed of surfactants, phospholipids, which permanently interact with all sorts fo polyelectrolytes, like proteins, to develop and transform themselves. In this way, the mixtures of polyelectrolytes and surfactants can represent model systems for the study of the interactions between proteins and membranes.For obtaining more performant the formulas based on polyelectrolytes and surfactants, depending on the applications, it is important to understand how the surfactants and polyelectrolytes interact when they share the same medium.
The polyelectrolytes and the surfactants of opposite charge can interact vey fast and form hydrophobe complexes in solution. These complexes can adsorb themselves at the interfaces water-air and water-oil, modifying considerably the foaming force (properties) of a solution. They can make the foam more stable then it would have been if the solution was composed of only surfactant molecules or polyelectrolytes.
The polymers and the surfactants are easily associated in the industrial formulae. Frequently the small amphiphile molecules bring their adsorption properties at interface and the macromolecules their rheological properties. When they coexist in the same medium, the two species interact and offer good suplimentary (auxiliary) properties to the system or the contrary can cause undesirable problems. This interaction can be translated in most of the cases as a specific association and the formation of new and original structures which can exhibit synergetic effects. An common example of such a structure associating the two species is that of membranes of all biological cells. These are composed of a complex assembly of particular polymers- proteins- and of surfactants- phospholipids.
Still, in the systems with a high ratio between the surface and the volume like foams or emulsio 18418y2411s ns, the interfacial phenomena play a leading role and are those that control most of the formation mechanisms. Being given the considerable importance of foams and emulsions in the industrial world, it is essential to understand, for mastering, the interfacial parameters which affect the stability of those systems, like the structure and composition of the adsorbed layers, the superficial tension, the surface viscoelasticity, the molecular interactions and the attractive or repulsive forces in the thin film.
Depending on the characteristic properties of a polymer, it can or can not be a good candidate for being associated with a surfactant for industrial applications. A particular interesting case is that where not only that the polymer develops interactions with certain amphiphilic molecules but it also is a surfactant itself. Also, if we want to favour one class of polymers, it may be suitable from the point of view of application, to choose a hydrosoluble polymer which allows the avoidance of the environmental and toxicological problems caused when using organic solvents. The solubility in water offers a wide range of applications in biology, medicine, in the hygiene field, foods and detergents industries. In certain cases, it may turn out as interesting to replace the polyelectrolytes with neutral polymers. En effet, les polyélectrolytes adsorbés fournissent des forces de répulsion électrostatiques de double couche souvent utilisées pour stabiliser des colloïdes.Of course, the polyelectrolytes adsorbed deliver electrostatic repulsive forces of double layer often used for stabilizing the colloids. Cependant, l'amplitude de ces forces à longue portée est considérablement réduite en présence de sel dans la phase continue, ce qui présente un inconvénient majeur pour beaucoup d'applications, en particulier biologiques, qui met en jeu des forces ioniques élevées. But, the amplitude of those forces on long distances is considerably reduced in the presence of salts in the continuous phase, which represents a major inconvenience for many applications, particularly the biological ones, which imply strong ionic forces.
The stabilization of foams is a major goal in the domains quoted previously (detergents, cosmetics, agriculture) but also for the controlled recovery of petroleum and minerals by "floating", or for the cleaning of the nuclear reactors. The foam may be used because it permits the utilization of a small quantity of active product in a large volume: at the cleaning of the nuclear reactors it is injected a foam containing very small quantity of water and detergent. After washing, the foam is recovered and destroyed, because it can become radioactive at treatment. Another peculiarity of the foam is the fact that it shows interesting mechanic properties, while they are composed of two fluids with low viscosity, water and air! This peculiarity is shown through the controlled recovery of petroleum: a non-negligible quantity of petroleum is recovered by injecting gas at high pressure in the well, but these operations have a small yield because the gas infiltrates the oil due its low viscosity compared to that of the petroleum. The injection of the foams allows that problem to be solved.
Polymer-surfactant complexes
We distinguish three types of couples polymer-surfactant where the two species interact between themselves in aqueous solution :
When the dominating forces responsable for the interactions are clear enough for the last two categories, the driving force for the interaction inside the system neutral polymer- charged surfactant is less obvious. So, those systems arouse a great interest from theoretical and experimental point of view.
The critical aggregation concentration and chain saturation
In a system with fixed concentration in polymer in which are added increasing quantities of surfactant, Jones defined in 1967 two critical concentrations of surfactant to which we refer as CAC and X2 [ Jones, 1967]. The CAC, critical aggregation concentration is concentration from which appear the interactions between polymer and surfactant. Agreggation has here the meanning of formation of surfactant agreggates, in other words micelles linked to the polymer. X2 is the surfactant concentration for which the polymer is saturated in surfactant. These two concepts are essential for understanding and studying the interactions polymer- surfactant and are illustrated by the share stress curves.
In figure I.8 are reproduced the classic share stress curves of SDS solutions for differnt concentrations of PVP as function of SDS concentrations [Lange 1971 ou Purcell]. The essential characteristics of those curves are similar to those obtained for the system POE-SDS [Cabane 1977, Cooke 1998] and thus they summarize as : In presence of polymer, the share stress curve presents the two transitions mentioned plus haut at concentration CAC and x2. the concentration CAC represents the starting point in the formation of polymer- surfactant complexes. From this concentration it can be observed a constant (plateau) surface tension which reflects a constant activity of SDS molecules. That signifies that the surfactant monomers added along the plateau are consumed to form agreggates linked to the polymer until the chains are saturated with surfactant.From x2, the activity is constant meaning that in the solution are formed free micelles no longer linked to the polymer.
En présence de polymère, la courbe de tension de surface présente les deux transitions mentionnées plus haut aux concentrations CAC et x
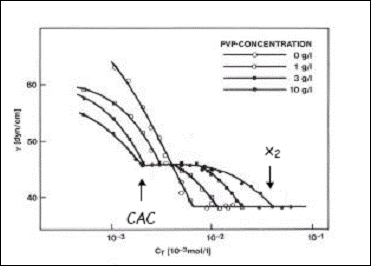
Figure I.8 : Surface tension of aqueous solutions of SDS for different concentrations in PVP [Lange 1971].
The figure I.8 shows that x2 increases with the concentration in polymer whereas the CAC is less sensitive to small quantities of polymer. In the case when the polymer itself if very tensioactive, as in the case of POP ( polypropylene oxide) and also PNIPAM, the results of surface tension for the mixed systems are difficult to interpret. La Figure I.8 montre également que x augmente directement avec la concentration en polymère alors que la CAC y est peu sensible sauf pour les très petites quantités de polymère.
Which is the driving force of the formation of complexes neutral polymers and hydrosoluble charged surfactant ?
Before taking an interest in the interaction polymer- surfactant, it is useful to consider first the self-assembly, namely the micellisation, of a classic ionic surfactant. The driving force for the assembly is the reduction of the contact area between water and the hydrocarbonated chains of surfactant. However, the micellisation results from a fragile equilibrium between many froces promoting or opposing the interaction. Particularly, the main force rezisting the agreggation is the electrostatic repulsion between polar charged heads when these last ones gather at the circumference of the micelles. The heavy density of charge resulting in that area will favorise the condensation of counter-ions to minimize the electrostatic potential and the repulsion between heads. Not only for geometrical restrains, it is important to keep the disposition ...........
Cependant, la micellisation résulte d'un équilibre délicat entre plusieurs forces favorisant ou s'opposant à l'interaction. En particulier, la force principale résistant à l'agrégation est la répulsion électrostatique entre têtes polaires chargées quand ces dernières sont rassemblées à la périphérie de la micelle. Ne serait-ce que pour des raisons de contraintes géométriques, il est important de garder à l'esprit que dans le modèle des micelles sphériques, la distance entre têtes polaires à la périphérie de la micelle est considérable à une échelle moléculaire. Une fraction de cette surface sera couverte de contre-ions mais une partie majoritaire sera constituée de chaînes hydrocarbonées exposées à l'eau, ce qui est une situation énergétiquement très défavorable (Figure I.9).
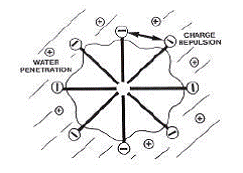
Figure I.9 : Représentation schématique d'une micelle de tensioactif anionique comme le SDS d'après Goddard [1993].
In consequence, all agents capable of reducing one and/ or other unfavorable interactions ( repulsions between the heads or great contact surface water- hydrocarbonated chains) will promote the micellisation. A first classical method is the introduction of salts which blocks the repulsions between heads and a second method is the addition of a cotensioactive substance of alcoholic type with short chain which will insert in the micelle.
Consider now a hydrosoluble neutral polymer composed of typical hydrophilic zones and hydrophobic regions. If the macromolecule is flexible enough, then it is possible to consider a configuration permitting at once a association ion-dipole between the ionic heads of the surfactant and the hydrophile part of the polymer chain and a contact between the hydrophobic regions of the polymer and the hydrocarbonated area of the micelle exposed to water. These configuration allows to block the electrostatic repulsions and diminish the hydrophobic area exposed to water. It is possible, due to the model of interactions between a neutral polymer and ionic surfactant developed by Ruckenstein et Nagarajan, to calculate the values of CAC of different systems from a certain number of parameters [Nagarajan 1979].
Structure des complexes polymère neutres-tensioactifs anioniques
La structure permettant de relaxer une partie des deux contraintes décrites envisagée par différents auteurs est appelée structure de chapelet. Elle est constituée d'une macromolécule décorée de micelles de tensioactifs (Figure I.10) [Shirahama 1974, Cabane 1977]. Cabane et Duplessix ont étudié en détails par diffusion des neutrons aux petits angles la structure de complexes POE-SDS [Cabane 1982, 1985 et 1987]. Ils en déduisent en particulier la stoechiométrie des chapelets ainsi que les différentes tailles caractéristiques associées tant du point de vue du polymère que du tensioactif.
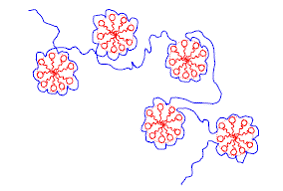
Figure I.10 : Représentation schématique de la structure de chapelets.
Remarques sur la formation de complexes polymère-tensioactifs
La formation de chapelets, c'est-à-dire de micelles de tensioactifs liées à la chaîne de polymère est énergétiquement plus favorable que l'autoassociation des molécules amphiphiles ce qui se manifeste dans une concentration d'agrégation critique (CAC) plus basse que la concentration micellaire critique (CMC).
Plusieurs conditions sont liées à la formation de la structure de chapelets :
cette structure nécessite des chaînes de polymère suffisamment longues avec un squelette flexible
plus le polymère est hydrophobe et plus l'interaction avec les tensioactifs aura lieu tôt à la fois en termes de concentration en tensioactifs et de longueur minimale de la chaîne de polymère. Parallèlement, les macromolécules très peu hydrophobes comme le PAM interagissent peu avec les tensioactifs.
l'association avec les tensioactifs est renforcée quand, à l'interaction hydrophobe, s'ajoute une interaction hydrophile. C'est le cas du PVP avec les tensioactifs anioniques car ce polymère présente un résidu de charge positive sur chaque monomère qui permet une attraction électrostatique forte avec les têtes polaires de charge opposée.

Figure III.1 : Représentation schématique des configurations adoptées par des chaînes de polymère adsorbées ou greffées sur une surface.
L'adsorption des polyélectrolytes est régie par les interactions électrostatiques et dépend donc très fortement des densités de charge de la surface et de la chaîne de polymère ainsi que de la concentration en sel. Ainsi, les polyélectrolytes fortement chargés s'adsorbent en quantités très faibles, voire pas du tout sur les surfaces neutres. L'addition de sel augmente l'adsorption. En revanche, les polyélectrolytes s'adsorbent sur les surfaces de charge opposée.
Le polymère agit comme un contre-ion géant pour la surface et l'entropie augmente considérablement quand la chaîne de polyélectrolyte unique remplace les petits contre-ions de la surface et relargue simultanément ses propres contre-ions. A faible concentration en sel, cette adsorption est très forte et la chaîne de polyélectrolyte se couche à plat sur la surface.
L'addition de sel réduit la force de l'interaction et peut provoquer la désorption du polymère.
Si la formation de ces complexes en volume a fait l'objet de nombreuses études, leur structure aux interfaces ainsi que les mécanismes d'adsorption et de stabilisation des mousses sont encore mal compris. Ce travail s'inscrit dans ce contexte et a pour but de décrier l'interaction des polyelectrolytes et des tensioactifs de charge opposée en surface. Notre approche a consisté a mener dans un premier temps une étude systématique afin d'identifier les paramètres qui gouvernent l'interaction polyelectrolyte - tensioactif en surface, et de déterminer dans quelle mesure ils permettent de modifier les caractéristiques de l'adsorption. Comme le montre la figure ci-dessous, nous avons principalement étudie le rôle de trois paramètres :
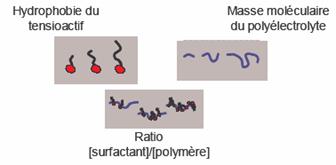
L'hydrophobie du tensioactif et le ratio des concentrations en tensioactifs et en polyelectrolyte sont deux caractéristiques fondamentales qui permettent de contrôler l'hydrophobie des complexes formés et donc leur solubilité ainsi que leur affinité pour l'interface eau-air. La masse moléculaire du polyelectrolyte est un paramètre qui influence la flexibilité des chaînes et leur capacité a complexé les molécules de tensioactifs.
Afin d'étudier ces paramètres, on a choisi de travailler avec un système bien caractérisé, compose d'un polyelectrolyte anionique de type Praestol et de tensioactifs cationiques. Et puis un polyelectrolyte cationique de type Praestol et de tensioactifs anioques. On trouve commercialement des Praestol de faible indice de polydispersité comprenant entre 1 et plusieurs milliers de monomères. De même, les tensioactifs utilisés existent sous une forme très pure.
Dans la première partie de ce travail, om a cherché a dresser une sorte de diagramme de phase de la surface. On a utilisé la mesure de la tension superficielle par al méthode de stalagmomètre. Comme l'adsorption de ces complexes est fortement conditionnée par leur structure en solution, on a également étudie l'interaction des polyelectrolyte /tensioactive en volume a l'aide d'une technique poténtiomètrique utilisant des électrodes spécifiques.
Dans la deuxième partie on a étudié les propriétés de rhéologie de ces complexes. Pour cela, on a utilisé deux techniques de rhéologie permettant d'étudier la réponse des solutions lorsqu'elles sont soumises a deux types de contraintes : les efforts normales et les contraintes de cisaillement. Les mesures de rhéologie en cisaillement ont été effectuées à l'aide d'un Viscosimètre à cylindres coaxiaux et un viscosimètre cône - plaque. Cette démarche on a permis de déterminer les propriétés rhéologiques des solutions en écoulement de cisaillement et en écoulement elongationel.
2. Tensioactive substances
The tensioactive substances, also called surfactants, or amphiphilic molecules are molecules which posses a hydrophilic head ( polar or charged), and a hydrophobic tail having weak solubility in water (Figure I-1).Due to that double solubility for the polar and apolar media, the the tensioactive substances adsorb themselves at interfaces. That is why they are used in the detergents formulae as lye wash (alkaline solution) or in the composition of dishing products to eliminate the grease; the hydrophobic part of the surfactant can attach to the stain of grease, hydrophobic material. After rinsing with water, the hydrophilic part is violently removed with water and together with the stain of grease. Those molecules equally used as foaming agent or as emulsifier: those molecules place themselves, preferentially at the boundary of water and the apolar media. This property allows them to stabilize the emulsions and the foams, which represents a major stake in the detergents and cosmetics fields.
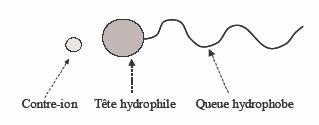
Figure - Structure of a tensioactive substance.
There are many kinds of tensioactive substances, which are of natural origin( phospholipids) or synthetic origin ( petroleum derivatives). Among them, we distinguish the charged tensioactive substances and the non charged tensioactive substances. The charged tensioactive substances posses a ionic head and a counter-ion of opposite charge, which dissociates in water. There are two types of ionic tensioactive substances: the cationic tensioactive substances, who's head carries a positive charge (and a negative counter-ion) and the anionic tensioactive substances, who' head carries a negative charge (and a positive counter-ion). Also there are zwitterionic tensioactive substances, which carry two opposite charges on the same molecule and the amphoteric tensioactive substances, who's charge can vary with the pH. As well, if the the most used tensioactive substances have a hydrocarbonated hydrophobic tail (composed of a succession of -CH2-), there are also tensioactive substances with siliconed (-SiO(CH3)-) and fluorinated (-CF2-). The chemical structure of a surfactant and the ratio of size and the solubility of the tail and of the head in water or oil determine its properties at the interfaces and in solution.
|